
Evolution in a Toxic World
How Life Responds to Chemical Threats
240 pages
6 x 9
10 illustrations

240 pages
6 x 9
10 illustrations
With BPA in baby bottles, mercury in fish, and lead in computer monitors, the world has become a toxic place. But as Emily Monosson demonstrates in her groundbreaking new book, it has always been toxic. When oxygen first developed in Earth's atmosphere, it threatened the very existence of life: now we literally can't live without it. According to Monosson, examining how life adapted to such early threats can teach us a great deal about today's (and tomorrow's) most dangerous contaminants. While the study of evolution has advanced many other sciences, from conservation biology to medicine, the field of toxicology has yet to embrace this critical approach.
In Evolution in a Toxic World, Monosson seeks to change that. She traces the development of life's defense systems—the mechanisms that transform, excrete, and stow away potentially harmful chemicals—from more than three billion years ago to today. Beginning with our earliest ancestors' response to ultraviolet radiation, Monosson explores the evolution of chemical defenses such as antioxidants, metal binding proteins, detoxification, and cell death.
As we alter the world's chemistry, these defenses often become overwhelmed faster than our bodies can adapt. But studying how our complex internal defense network currently operates, and how it came to be that way, may allow us to predict how it will react to novel and existing chemicals. This understanding could lead to not only better management and preventative measures, but possibly treatment of current diseases. Development of that knowledge starts with this pioneering book.
"The book should appeal to a broad audience and will spur future interest in the fields of evolutionary toxicology....well-written"
Ecology
"This book is utterly fascinating, covering all sorts of toxins from the beginning of life on Earth, and continuing through modern times and predicting what the future may hold."
The Guardian GrrlScientist blog
"Exceedingly detailed and thoroughly researched treatise… Monosson's prose is necessarily complex as she gives her subject its scientific due, but for those who seek a thorough understanding of this timely issue, she offers a text solidly grounded in both history and contemporary analysis. Patient readers will be well rewarded."
Booklist
"this book really made me love evolutionary biology"
Toxicology Student Association blog
"...it does provide detailed information about how life evolved to survive everything that our planet, other organisms and its own cells can throw at it through some fascinating examples."
Science Illustrated
"Monosson posits that the field of toxicology should look to evolution to understand biological responses to today's chemical threats."
Conservation
"A toxicologist traces how life evolved to deal with toxic substances and how this plays into chemical exposures today."
Science News
"The book is written in an accessible style and is aimed at the general public, as well as at scientists. The third-person scientific writing is interspersed with personal anecdotes and thoughts, which should help to make the book more appealing."
Bioscience
"Evolution in a Toxic World addresses the challenges posed to life on earth by a plethora of toxic threats. There are chapters dedicated to ozone, oxygen, metals, assorted chemical agents, cancer, etc. ... The book serves as an excellent introduction to the topic of toxicology and evolution for the college student or general reader."
Science Books & Films
"This 222-page book is a thought-provoking summary of an important but often ignored subject matter, environmental toxicology."
Barney Lipscomb, Journal of the Botanical Research Institute of Texas
"Evolution in a Toxic World describes how biological defenses have evolved over time, responding to challenges of an ever-changing environment. Monosson draws on 'lessons learned' to address the key question of whether we can defend ourselves against the myriad agents that we introduce into the environment. In this engaging and sweeping book, she looks for the answer."
Jonathan M. Samet, Director, Institute for Global Health, University of Southern California
"Dr. Monosson has provided an original synthesis of this intriguing and often neglected topic. She uses fascinating examples to illustrate how an evolutionary perspective enriches our understanding of chemicals and their myriad interactions with living systems."
Mark E. Hahn, Senior Scientist, Woods Hole Oceanographic Institution
"An intriguing and thought-provoking synthesis of how environmental stressors have shaped life on the planet. The book challenges us to consider that genomic diversity not only tells us about the past, it helps us predict the future: how different groups of organisms will respond to new environmental stressors, including those of human origin."
L. Earl Gray, Research Biologist
"Well written, very readable, thought-provoking. Highly recommended reading for environmental scientists and non-scientists."
Peter M. Chapman, principal, Golder Associates Ltd
"A new field is born—evolutionary toxicology—and Monosson has opened the door for us."
Quarterly Review of Biology
Preface
Acknowledgments
Chapter 1: An Introduction
PART I. ELEMENT
Chapter 2. Shining a Light on Earth's Oldest Toxic Threat?
Chapter 3. When Life Gives You Oxygen, Respire
Chapter 4. Heavy Metal Planet
PART II. ANIMAL
Chapter 5. It Takes Two (or More) for the Cancer Tango
Chapter 6. Chemical Warfare
Chapter 7. Sensing Chemicals
Chapter 8. Coordinated Defense
PART III. HUMAN
Chapter 9. Toxic Evolution
Chapter 10. Toxic Overload?
Appendix: Five Recent Additions to the Chemical Handbook of Life
Notes
Selected Bibliography
Index
 Methicillin-resistant Staphylococcus aureus (MRSA), in green, with human cell, grey. Photo by NIAID, used under Creative Commons licensing.
Methicillin-resistant Staphylococcus aureus (MRSA), in green, with human cell, grey. Photo by NIAID, used under Creative Commons licensing.
Reposted from Whole Terrain with permission.
We are a chemically-addicted society. I am not talking caffeine, alcohol, or painkillers–although there is plenty of that–but chemicals designed to kill. Of the nearly 37 million pounds of antibiotics used in the United States each year, some 7 million pounds are ingested or injected, aimed at whatever ails us: staph, strep, salmonella, syphilis. The rest is used by the agricultural industry.
But that’s a pittance compared with the more than 600 million pounds of pesticides directed at plants and animals infesting farmlands, backyards, and homes. I imagine a pile of these so-called pests: the boll weevils, corn borers, gypsy moths, bed bugs, horseweeds, pigweeds, fish, rats, and mice poisoned over the past 60 or 70 years, our golden age of pesticides, and conjure an image of a great pyramid. Then add all the bacteria, harmful and beneficial alike, killed by antibiotics.
As a toxicologist, I am fascinated by the relationship between toxic chemicals and evolutionary processes. Recently, I’ve been thinking about chemicals, death, and the resilience of life, particularly in those species we try hardest to control. And so next to that pyramid of death, I imagine another smaller, but growing, pyramid of all things resistant. Like beings from some alternate toxic universe, these are descendants of species specifically targeted for obliteration by insecticides, herbicides, and antibiotics. I imagine them feasting upon our farm fields, indulging on the arms and legs of children, and threatening to return us to the pre-antibiotic era.
On corn, soy, and wheat fields across the country, in hospitals, hotels, and homes in our communities, life is evolving. Rapidly. This is life sprung from the trap of chemical death. Nature is schooling us big time. We would do well to take note.
In little over a century, we have squandered one of our most valuable defenses against pathogenic bacteria, namely antibiotics, and there is plenty of blame to go around. Many people contribute to the rise of antibiotic-resistant superbugs, whether they are doctors pacifying anxious parents, those of us who don’t follow doctor’s orders, the agricultural industry preventatively treating disease and encouraging growth in livestock, or hospitals fending off recalcitrant infection. The most infamous outcome of this unbridled chemical warfare is the evolution of methicillin resistant staph, or MRSA: the poster-bug for resistance. Of the roughly 80,000 Americans who become infected with MRSA each year, an estimated 11,000 will die. Likewise, a once-curable pneumonia recently killed seven patients at a well-regarded national hospital. Totally drug-resistant tuberculosis has surfaced in India, Italy, and Iran. Gonorrhea is on the verge of shaking free from its vulnerability to antibiotics. Each of these new superbugs ought to strike fear into all of us, because one way or another we are all within their reach.
Bacteria may be among the most primitive life forms on earth, but they have proven to be formidable opponents with an impressive arsenal of detoxification genes spread around their collective genome. Even bacteria collected deep within caves well beyond the reach of industrial or pre-industrial age chemicals harbor genes for detoxifying our modern antibiotics. Since the majority of our antibiotics are derived from nature, these are chemicals with which bacteria have had a long history.
 A sample of penicillin mold presented by Alexander Fleming in 1935. Photo by the Science Museum of London, used under Creative Commons licensing.
A sample of penicillin mold presented by Alexander Fleming in 1935. Photo by the Science Museum of London, used under Creative Commons licensing.
Penicillin, derived from mold, became one of the world’s first miracle drugs. Yet just a couple of years after its introduction, it began to fail. But those were the booming days where industrialized countries enjoyed “Better things for better living…through chemistry” thanks to DuPont and other chemical industry giants. There was certain to be a technological fix. A new antibiotic would be developed or the chemical structure of penicillin could be tweaked. Man would not be conquered by Nature. In hindsight it is not surprising that new antibiotics, many cut from the same biological cloth as penicillin, eventually succumbed to resistance; it had been lurking in the bacterial gene pool for millennia. We simply hit them with a dose of their own medicine. Resistance was predictable, and perhaps even avoidable had physicians and the public alike heeded early warnings. More than six decades into the Golden Age of Antibiotics despite ongoing research and development, physicians, and public health scientists warn that we are heading toward a pre-antibiotic future. And antibiotics aren’t the only industrial age chemical rendered useless by Mother Nature.
DDT was another miracle chemical, controlling lice, fleas, mosquitoes, and bed bugs in an age when there were few options to prevent deadly insect-borne diseases. Giddy with the promise of synthetic chemicals, one early twentieth century zealot envisioned the day when pesticides like DDT would banish “…all insect-borne disease from the earth.” By 1972, over one billion pounds of the chemical had been applied to homes, gardens, wetlands, and millions of acres of US cropland. Books like Silent Spring and Who Really Killed Cock Robin revealed to the general public the pesticide’s toxicity to birds, while houseflies, lice, mosquitos, and agricultural pests were evolving resistance.
The rise of resistance is far from unique to bugs and bacteria though. Another post-world war savior was the herbicide 2,4-D, which targeted broad-leaved plants, leaving grasses like corn alone. Yet, a little over 10 years after 2,4-D went to market, weeds resisted. Today there are over 100 different herbicides, and over 200 resistant weed species. In 2010, weeds resistant to just one herbicide infested over 33 million acres of crop land in the U.S. Today the percentage has more than doubled. By the time you read this, the acreage of cropland infested with herbicide resistant weeds will likely be higher. If there is an herbicide, you can bet there are resistant weeds.
“Agriculture,” wrote plant scientist Jonathan Gressel back in 2009, “is the largest evolution laboratory presently on earth today with herbicides as the most ubiquitous man-made artificial selector for evolution on the planet.” In 2012, herbicide resistance loomed so large that the National Academy of Sciences, the premier scientific organization in the U.S., gathered together the top agricultural scientists for a summit on herbicide resistance. One goal was to prevent the herbicide Roundup, the “once in a century herbicide,” from going the way of penicillin.
If there is somewhat of a silver lining to these toxic clouds, it may be that rapid evolution isn’t just for pests and pathogens. Bacteria, insects, plants, and even vertebrate species are evolving in response to chemical pollutants, including metals, PCBs, and road salts. Fish, frogs, and salamanders have all been found living and breeding, while not necessarily thriving, in highly contaminated ponds and rivers. Recently, after a presentation about environmental contaminants and evolution an audience member asked, “If we can’t rein in all of these chemicals, why bother? Why not let nature take its course?” I should have said “Because we just don’t want to live in that kind of world. I don’t want my kids and their kids to live in that world. We can, we must, do better.” Instead I gave some impersonal scientific answer about disrupting the web of life. Later, I asked a couple of evolutionary biologists what they thought about the rapid evolution of wild species in response to human activity. “I think it’s sort of hopeful,” one quipped. “Populations will survive no matter what we do.” The other agreed that it was sort of like Nature thumbing her nose at us. But the truth is, we know very little about the long-term implications of evolution in response to pollution.
Evolutionary dogma holds that, like cash-strapped cities, there is very little excess energy in life’s budget. Should there be any, it is put into reproduction and development, thus optimizing fitness. When a population adapts, whether to sudden shifts in available food or to pollutants, energy may be diverted from reproduction to defense, which burns through valuable energetic resources. So some species take a different tact. Consider the yellow perch inhabiting metal contaminated lakes in the Rouyn-Noranda region of Quebec. Decades of copper smelting left the lakes contaminated with toxic metals like zinc, cadmium, and copper. Studies show that over the course of some 50 years, populations of yellow perch evolved. Yet, rather than spending precious energy on detoxification–populations survive by maturing young and spawning early. In other words, these perch live fast and die young.
Piles Creek, New Jersey offers another scenario. Contaminated with mercury along with a hefty load of other industrial pollutants, it is a system that upon first glance seems normal, and it is perhaps even comforting that life can indeed survive even in such a grossly-polluted site. There are killifish, crabs, and shrimp as one might expect in a tidal creek. Yet the system is anything but normal. Instead, it has become a topsy-turvy world full of chemically-addled survivors. The shrimp, normally hunted by the killifish, are fat and happy, once-predatory crabs now feed on detritus, mud, and algae. Meanwhile the hunters are more easily hunted, as killifish from the creek have become less efficient at capturing prey and avoiding predators. It is an ecosystem that is resistant yet sickly.
There are secondary impacts too. For all those species that adapt to their polluted environment, there is very likely a predator that has not. So while fodder fish have apparently evolved free of cost, as is the case for PCB and dioxin-adapted killifish and tomcod, their predators–the bluefish, kingfisher, and mink–are picking up the bill. Along the banks of PCB-contaminated waters, mink populations in particular carry around enough of the organochlorine to cause reproductive failure.
As a toxicologist, I used to focus on the toxic effects of chemicals. Did they kill? Reduce reproduction? Slow growth? Over the past half-century, toxicity testing has managed to flag some of the most egregious toxicants. And, we are in a far better place today than we were in the 1960’s when our chemicals drove raptor population towards extinction. But now I see some of the more insidious effects of toxic chemicals. Over the course of 3.5 billion years, life made its peace with the countless naturally occurring toxic chemicals. Yet in the blink of an eye, we have added hundreds of thousands of new chemicals to the mix and continue to do so at an alarming rate: antibiotics, pesticides, and industrial chemicals. We are challenging Nature to a game of evolve or die, and it is a game we will surely lose. In the closing paragraphs of Silent Spring, Rachel Carson lamented that insecticides were, “As crude a weapon as the cave man’s club, the chemical barrage has been hurled against the fabric of life–a fabric on the one hand delicate and destructible, on the other miraculously tough and resilient, and capable of striking back in unexpected ways.”
We cannot turn back the clock, nor would most of us want to return to pre-industrial, pre-antibiotic days (and Carson did not advocate this either). But we must learn how to live in balance with the rest of life: to manage pests without creating superbugs, to protect individuals from disease without inviting epidemics, to benefit from technology without threatening the health of future generations. Our chemicals are powerful. They can influence the course of evolution in wild and unpredictable ways–often not to our benefit. It is time we reduce the pressure. Our lives and the lives of those we hold dear, may well depend upon it.
With more and more cases of Zika virus being reported, some experts are calling for the use of DDT to combat the mosquitoes that transmit it. We asked Emily Monosson, author of Unnatural Selection and Evolution in a Toxic World to weigh in on the proposed use of pesticides, and what else we can do to protect ourselves from disease-carrying insects.
Over the past fifteen years I’ve experienced the spread of Lyme disease in my own backyard. Within a matter of years, the field where my daughter used to unpack her little wicker basket—laying out a blue-checked table cloth and two teacups painted with images of Winnie-the-Poo and Eeyore—has transformed from a field of emerald green grass and wildflowers to a danger-zone where the consequence of a Sunday afternoon tea-party may be a bout with Lyme.
In little over a decade, the Lyme bacterium, the deer ticks, the white-footed mouse, and deer that host the ticks have all become more prevalent. This is our new reality. Human-induced change from altered habitats, reductions in predators, and a warming climate are enabling pest and pathogen to move into new spaces. And Lyme is a harbinger of things to come.
Mosquitoes, along with their disease-causing hitchhikers like West Nile, Equine encephalitis, Dengue, and now Zika, are on the move, finding new habitats and naïve populations ripe for infection. Just as Lyme has made tick experts out of us all (no, that one is just a dog tick), we are on a first-name basis with mosquitoes like Aedes and Culex. Here in the Northeast dozens of mosquito varieties bite, buzz, and mate. Some inject pathogens, most don’t. Each has their own preferences and habits. Culex pipiens carries West Nile and favors biting birds to humans; Coquillettidia perturbans is a midsummer’s carrier of Eastern Equine Encephalitis and feeds upon anyone and everyone from birds to humans; Ochlerotatus sticticus is another daytime disease carrying biter. Others prefer non-human mammals, or frogs, or even snakes. In Massachusetts alone there are over fifty varieties of mosquitoes. Of all disease-carrying insects, mosquitoes are the hands-down winners as the world’s greatest menace. But, of the thousands of known varieties worldwide, only a few hundred bite humans, and fewer transmit disease. Then again, it only takes one.
The Asian tiger mosquito, Aedes albopictus, is a relative newcomer to the Northeast. First identified nearly 30 years ago in a Texas dump, the aggressive blood-sucker, likely aided by a warming climate, has marched northward over the decades. Known to transmit as many as twenty different kinds of pathogens in different regions around the globe, health experts fear that someday soon the tigers will be delivering pathogens like Dengue, chikungunya, or Zika to more northern regions of the U.S.

The Asian tiger mosquito, Aedes albopictus is known to transmit as many as twenty different kinds of pathogens in different regions around the globe, including the United States. By James Gathany/CDC, via Wikimedia Commons
Zika and Dengue are also transmitted by Aedes aegypti. Warmer-weather mosquitos which, like the tiger, are particularly well adapted for life amongst humans with a penchant for laying their eggs in roadside bottle-caps, abandoned tires, or backyard tarps. A habit that makes them particularly successful breeders—free from natural predators like fish or dragonfly larvae—and difficult to eradicate with pesticide spray programs. Centuries ago, yellow fever outbreaks in Northeast cities from Boston to Philadelphia suggest that Aedes, likely carried aboard ship from far-flung ports, survived long enough to spread disease before succumbing to cool weather. While these beasts of the subtropical wilds haven’t yet made a go of it in the north, climate change may alter that.
Those of us who remember mosquito-free evenings of the 60s and 70s probably also remember the fog of DDT trucks. With the advent of synthetic chemistry, we turned to miraculous chemical cures like DDT and other powerful pesticides. It seemed there wasn’t a pest we couldn’t conquer. A few decades later our hubris was rewarded with a catastrophic decline in raptors and resistance in targeted insects. Plenty of communities still spray next-generation pesticides (like resmethrin and biologically-based bacterial sprays) but for every chemical cure there is, or will likely soon be, a resistant population. Pests with amped up detoxification systems or target sites with reduced sensitivity. Not to mention harm done to the innocent bystanders, beneficial insects that prey upon pests or pollinate plants. And, for a species like Aedes, pesticide from a large-scale spray program may not find its way into that bottle cap, or dog bowl or old tire. For these buggers, control requires a more personal touch: a weekly scan of the yard, junk yard, or parking lot. With a flight range of a couple hundred meters, clearing a yard or a neighborhood block of mosquito breeders can go a long way towards control.
So far, mosquitos carrying Zika haven’t yet been reported in the continental U.S.; though it is likely only a matter of time. And if it’s not Zika, some other mosquito-borne virus will be coming to our neighborhoods someday soon.
There is some hope. Most notably, scientists have engineered mosquitoes to produce offspring destined to die before they are old enough to reproduce. This strategy is already in trials in Brazil, Florida, and elsewhere. Because reproduction is the Achilles heel of the evolutionary process (inheritance, inheritance, inheritance!), it is a strategy unlikely to be circumvented by evolved populations. And while we have learned time and again that it is difficult to fool Mother Nature, we also need to consider the consequences of actually doing so. Like, the consequences of world without Aedes or other mosquitoes. Most ecologists aren’t too concerned. Predators that feed on mosquitoes or larvae will find other food; other pollinators will fill in where mosquitoes left off. But the end of mosquitoes isn’t in our near future; at best the strategy may work on localized populations or regionally rather than going global.
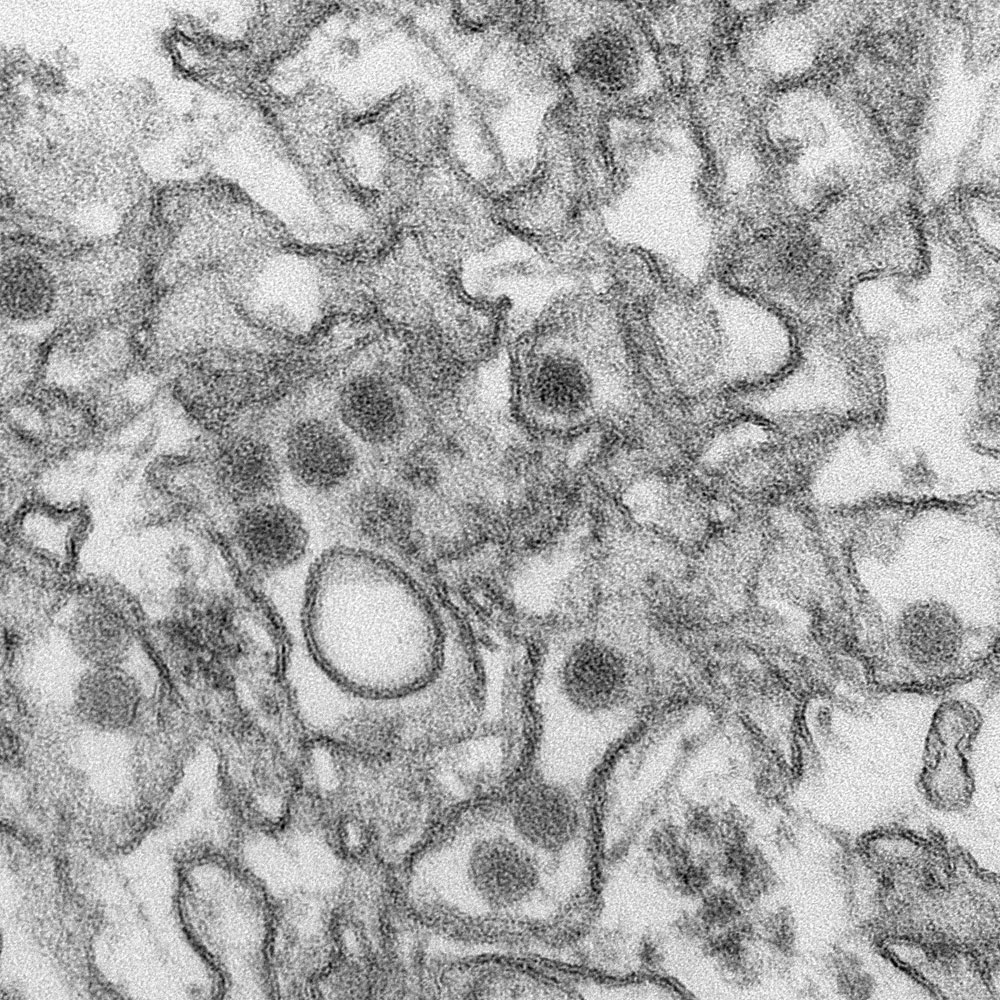
Transmission electron micrograph of Zika virus. By CDC/Cynthia Goldsmith, via Wikimedia Commons
On the flip side, if we can’t stop the pest, perhaps we can stop the pathogen. Mosquito-borne diseases have killed hundreds of millions of people (approximately one million individuals each year). Vaccines have saved many more. There aren’t yet vaccines for West Nile and chikungunya (though a vaccine for Dengue, twenty years in development, just became available in some countries), and of course Zika is prompting vaccine developers to scramble, promising accelerated vaccine development and production. Even so, it may be years before vaccines enable us to shed our long sleeves and ditch the mosquito repellent.
So what are our options? After nearly a century of resting on our chemical laurels, we need to think differently. There is no quick fix. As with Lyme we will all need to become a little more wary, a lot less cavalier and a bit more humbled by Nature. We will need to be more strategic about how and when we use pesticides, how we dress when we go outside, and perhaps even when we go outside. We also need to be more aware of our contribution to the problem. But that doesn’t mean we need to cloister ourselves indoors.
When we venture out into the field during tick season (a depressingly longer stretch of time each year), we expect ticks. Years ago we joked about “tick-checks.” Now they are a part of the daily routine. We scoffed at “birding couture:” the long sleeves, light clothing, pants tucked into socks. Not so now. Maybe it’s time to dig out that bug head net I bought for gardening but never wore. Not so comfortable, but what the heck. And sweeping around the yard every few days drying out the mosquito breeders will definitely become part of the spring and summer routine. As much as I know not to leave standing water, the dog bowl has nurtured plenty of newly hatched mosquitos over the years. With our ever-changing world, we don’t have to live in fear, but we do need to live more aware.
This post originally appeared on Emily Monosson's blog Evolution in a Toxic World and is reposted here with her permission.
We were closing in on the end of a glorious spring weekend when my husband discovered the bag. “Any chance you left this lying around — empty?” he’d asked holding the remnants of a one pound bag of Trader Joe’s raisins I’d purchased just the day before with images of molasses filled hermit cookies in mind. I hadn’t, nor had I made the hermits, or chewed away the corners of the bag. Apparently Ella (pictured below) had consumed every last raisin, save the two handfuls my husband snacked on before leaving the bag on the living room floor.
“I bet she won’t be feeling too good later,” he’d said, eyeing the ever expectant dog sitting at our feet, tail wagging, hoping for a few more of the sweet treats. He had no idea. Nor had I. Not really. I’d had some inkling of a rumor that raisins and grapes were bad for dogs, but never paid too much attention. It’s one of those things you hear at the same time you hear of people treating their dogs to grapes. So, to be safe (and feeling a bit sheepish that, as a toxicologist I ought to have an answer to the raisin question) I suggested he call the vet. And that is when we fell into the raisin hell rabbit hole. Five minutes later dog and husband were on their way to the doggie ER, pushed ahead of the mixed breeds and the Golden and the sad-sack blood hound and their people waiting for service.

Meanwhile I took to Google. Was this really a life or death dog emergency? If so, why weren’t we more aware? I get it, that one species’ treat can be another’s poison. Differences in uptake, metabolism, excretion. Feeding Tylenol to cats is a very bad idea (as if you could feed a cat a Tylenol tablet). And pyrethrin-based pesticides in canine flea and tick preventions are verboten in felines. The inability to fully metabolize and detoxify these chemicals can kill a particularly curious cat. But raisins in dogs? Not so clear. Googling will either send you racing off to the vet or to bed. You may even toss your best friend a few grapes for a late night treat, smug in the knowledge that those who have bought into the hysteria are hemorrhaging dollars while paying off the vet school debt of a veterinarian who is gleefully inducing their dog to vomit, while you snooze.
Even Snopes the online mythbuster was confused (though they suggest erring on the side of caution.)
By the time I arrived at the clinic, uncertain enough to follow up on husband and dog, Ella’s raisin packed gut under the influence of an apomorphine injection (a morphine derivative which induces vomiting in seconds) had done its thing. While Ben and I waited for Ella’s return in the treatment room, somewhat relieved, we played, “Guess how much?” Treatment with a drug, time with the vet, multiplied by the “after hours factor” this being a Sunday evening after all, we’d settled on something in the $300-400 range.
“Ella did great,” said the vet tech who’d taken her from Ben and hour or so earlier. “A pile of raisins came up. Some were even still wrinkled!” Phew. Potential disaster averted. We’d accepted that it’d likely cost a few hundred – but we’d soon be heading home with Ella in the back seat. We had a good laugh about the revisit of the raisins. But the vet tech wasn’t finished. That was just the first step. “So now we’ll give her some activated charcoal,” she continued “and you can pick her up on Tuesday.” Total estimated low-end estimate? A bit over $1000. Paid up front (I have wondered what would have happened if we couldn’t pay – but that is a whole other issue). Apparently we had underestimated the price of a good vomit.
Continue reading the full post on Evolution in a Toxic World.
This blog originally appeared on Emily Monosson's blog and is reposted here with permission.
The New York Times article, Doubts About the Promised Bounty of Genetically Modified Crops rightly argues that herbicide resistant GMOs haven’t reduced the use of chemicals on the farm. But by equating genetic modification with herbicide resistant plants, this article is misleading. Many of us agree there are problems with herbicide resistant plants, and that misuse, overuse and eventually resistance in weeds has put users on a toxic herbicide treadmill.

But GMO technology isn’t just about herbicide resistance. It can also be deployed to reduce pesticide use and, there are new ways to engineer plants and animals that don’t mix and match genetic material from vastly different species. Take potatoes engineered to resist one of the most destructive potato pathogens, late blight, the disease that devastated Irish potato crops and kicked off the Great Famine. When blight strikes it can destroy crops so rapidly that growers often use multiple applications (sometimes more than a dozen a season) of toxic fungicides to prevent disease. A decade ago 2000 tons of fungicide were applied to potato crops just here in the states. In turn, blight has evolved to resist many of those pesticides. For such an aggressive disease, genetic engineering may be one of the few options for growers wishing to reduce their use of toxic chemicals. In this case rather than inserting genes from a totally foreign species, one approach is to insert disease resistance genes from a more resilient relative.

The potatoes recently engineered by scientists at The Wageningen University Research in the Netherlands for example are, they say, indistinguishable from the potatoes we love to bake and fry. Why use engineering when a grower might breed for resistance, an ages old practice? One reason is speed. Engineering enabled the production of a disease resistant crop in three yearsrather than three decades. And unlike transgenics like Roundup Ready and Bt crops which introduce foreign genes onto an unfamiliar “genetic landscape” of the target species – the GMO that everybody loves to hate – these so-called cisgenic potatoes introduce new traits into familiar territory, reducing concerns for unintended consequences. Additionally, Wageningen University Research retains the intellectual property and offers non-exclusive licenses to parties interested in working with the genes or resistant plants in an effort to thwart corporate control. The cisgenic process along with other techniques like gene editing are providing opportunities for genetic engineering that call for reevaluation. Genetic engineering is a technology, not a product.
Below: an image from the Hartsfield-Jackson airport. Couldn’t resist, check it out next time you’re there.

This blog originally appeared on Emily Monosson's blog and is reposted here with permission.
Yesterday I read of a meningitis B outbreak at Oregon State University. Today, it’s the University of Massachusetts in Amherst, MA. MenB is a potentially lethal and easily spread infection particularly in settings where young adults gather together. As the university races to vaccinate tens of thousands of students, my thoughts turn to our daughter, a senior in college. A few years ago after writing a book chapter that included the history of meningitis B and the recent development of a vaccine, I had asked my daughter’s pediatrician (ironically in Amherst, MA) if she could receive the vaccination as she headed off for her sophomore year. They would not, stating that it was available only for those who had other health indications. Perhaps if it were more easily available, colleges would not have to react, and students would already be protected.
Below is an excerpt from that chapter about meningitis and the vaccine:
My father had just returned from the Navy, an apple-cheeked mischievous twenty year-old looking forward to his junior year in college when meningitis struck. It was 1946 and the last thing he recalled was brushing his teeth at home in the bathroom. For the next ten days he lay unconscious in a hospital bed his body fighting off an invisible army of bacterial invaders. Aided by the new miracle drug, penicillin, he survived, but not entirely unscathed. Shortly after recovery my father was jolted by brain seizures – his brain permanently damaged by the infection. For the remainder of his life he managed the condition with a combination of powerful antiepileptic drugs (while baffling his doctors by referring to the electronic brainstorms as a “free high.”)
Meningitis is a catch-all term for swelling of the tissues surrounding the brain and spinal cord. Specific viruses, fungi, and injury can all cause the potentially fatal condition but one of the most frightening and lethal causes is bacterial infection. Bacterial meningitis, caused by a handful of bacteria (Haemophilis influenza type b (Hib) or Streptococcus pneumonia and Neisseria meningitides) can kill in within a day, is often incurable, and may leave survivors with amputated limbs, hearing loss or brain seizures. My father was relatively lucky. One of the more intractable causes of meningitis is Neisseria, a bacterium adept at spreading through populations gathering together for the first time: freshmen dorms, summer camps, day care, the military barracks. Some five to twenty percent of us carry Neisseria in our nose and throat and unwittingly spread it around to those we share a meal, or a drink or a kiss. Most of us won’t get sick. A few of us may die from the infection, even today.
My kids were born in the 1990s. By the time they toddled off to school, they had received a slew of vaccines: measles, tetanus, mumps, polio, smallpox, chicken pox and even Haemophilus influenza and Streptococcus pneumonia (two other important causes of meningitis). But an effective vaccine against Neisseria meningitidis had not yet made it on to the recommended vaccine schedule. Then in 2005, just as they were heading off to the middle school milieu of new students, sweaty locker rooms, team sports and shared drinking bottles, a vaccine against a collection of N. meningitidis serotypes become available. Though the disease is rare here in the U.S.,compared to sub-Saharan Africa, in the so-called meningitis belt, I felt relieved. One more disease they wouldn’t get. Except.
Except for the escape artist, a serotype called meningitis B or MenB. Though rare, the infection that can take a turn for the worse within hours, has frustrated vaccine makers for decades. And it seems to pop up out of nowhere. In 2013 an outbreak at the University of California caused a freshman lacrosse player to undergo amputation of both feet. Four other students were infected, and the university was forced to provide prophylactic antibiotics to five hundred students. The next year an outbreak at that began at Princeton University caused the death of a Drexel student. In the first months of 2016, MenB hit three different colleges and killed one employee. Even in our “golden age of disease prevention,” and vaccine development, MenB has remained intractable through its ability to evade immunity.
It does this by wrapping itself in a sugary polysaccharide sheath that is identical to human polysaccharide molecules. Immune cells recognizing this molecule would have been naturally eliminated or deactivated as a protection against autoimmunity. By sequencing the pathogen’s genome vaccine makers have been able to discover antigenic proteins that would otherwise be hidden; four different antigens found on the majority of circulating Men B (a single pathogen may have several different circulating strains.) The discovery was a breakthrough for vaccine development. When Mariagrazia Pizza and co-workers reported their findings in the journal Science, they wrote: “In addition to proving the potential of the genomic approach, by identifying highly conserved proteins that induce bactericidal antibodies, we have provided candidates that will be the basis for clinical development of a vaccine against an important pathogen.” A few years ago when meningitis broke out at Princeton and UCSB campuses, the vaccine, licensed in Europe in 2013 but not yet here in the U.S., was offered to students on both campuses. One headline blared “California students to receive unlicensed meningitis vaccine.”
Sold as Bexsero by Novartis the vaccine (along with another new vaccine called Trumenba) was finally licensed in the U.S. in 2015. Hopefully it will become more widely available.
For more from CDC see here
On the fourth of July, 1985, as the sun shone and the temperatures rose, people celebrated by eating watermelon. Then they got sick — becoming part of one of the nation’s largest episodes of foodborne illness caused by a pesticide. The outbreak began with a few upset stomachs in Oregon on July 3; by the next day, more than a dozen people in California were also doubling over with nausea, diarrhea, and stomach pain. A few suffered seizures.
All told, the CDC estimated that more than 1,000 individuals from Oregon, California, Arizona and other states, along with two Canadian provinces, became ill from eating melons, picked from a field in California, contaminated with a breakdown product of aldicarb — one of the most toxic pesticides on the market. There were the usual calls for the pesticide to be banned. While it was eventually scheduled to be phased out, now the chemical is back — albeit with more restrictions on its use.
Banning a pesticide is tricky business once it’s made its way onto the market and into fields and orchards. Consider the Environmental Protection Agency’s flip-flopping on a ban on the insecticide chlorpyrifos. In 2000, the EPA deemed chlorpyrifos too dangerous for home use, and allowances for food residues were reduced as well. Yet it remained popular — and legal — for agricultural use. In 2015 the agency concluded the chemical was too toxic to use on our fruits and vegetables. Then in 2017, the EPA flipped, granting the insecticide, introduced and widely sold by Dow Chemical, clemency. Finally, last Thursday, in a decision lauded by environmental groups, a federal court nullified the agency’s decision and ordered chlorpyrifos to be banned within the next two months.
In its ruling, the court did what the EPA wouldn’t, stating that there was no justification for maintaining “a tolerance for chlorpyrifos in the face of scientific evidence that its residue on food causes neurodevelopmental damage to children.”
For many, it’s hard to fathom why such a decision couldn’t have been reached years ago. After all, the EPA is directed to consider the costs and benefits of chemical use on the environment, and the potential health impacts on humans weigh heavily in the decision — particularly when establishing how much pesticide may remain in our food from fruit to grains, the exposure must be deemed “safe.” Though we might not recognize it, EPA regulations are currently responsible for protecting our daily safety in numerous ways. Every time we choose conventional foods over organic, for example, we are putting our trust in the agency’s decisions. Often, that trust is warranted. But recent advances in toxicology, the workhorse science of the EPA, suggest that regulations based on earlier testing may not protect consumers from harmful exposures.
Indeed, today’s toxicologists are finding adverse effects that their earlier counterparts could only have imagined. One striking recent discovery, for example, showed that pesticides and industrial contaminants can impact not only the individuals initially exposed, but also their offspring, grandoffspring, and possibly even great-grandoffspring. Another demonstrated how chemicals like bisphenol A — or BPA, used to make some plastics — causes subtle but irreversible damage to developing brains.
But changing regulations to reflect these new findings is complicated; as is evident from the corporate interests in chlorpyrifos, any new law has complex social, political, and economic impacts, making it a high stakes game. Chemical regulation and testing requirements simply can’t keep pace with science. Take for example, testing for chemicals like BPA. These so-called hormone-disrupting chemicals were known to be problematic since before the turn of this century, yet approved tests for these chemicals have only recently emerged.
Recognizing that standard toxicity tests may lag behind the science, the EPA reviews registered pesticides every 15 years or so, providing an opportunity to reconsider old chemicals in light of more recent data. New findings of toxicity can also prompt a review, which is what happened with the insecticide chlorpyrifos. A series of epidemiological studies suggested the chemical had adverse impacts on children’s brains, causing the Pesticides Action Network North America and the Natural Resources Defense Council to circulate a petition prompting an EPA review. In 2015, the agency determined that, based on the new science, chlorpyrifos was so toxic that no trace of the chemical should remain on fruits and vegetables — essentially an all-out ban.
Over the past few decades, multiple studies have shown that other chemicals known to be neurotoxic, like mercury and lead, can alter brain development and impact children’s behavior. As a result, acceptable thresholds for exposure to these chemicals have also been lowered. Previous toxicity tests on animals often failed to reveal these kinds of impacts, in part because, somewhat obviously, lab rats aren’t children. “We’re using behavioral paradigms that aren’t exactly the same,” Deborah Cory-Slechta, a neurotoxicologist at University of Rochester who studies both lab animals and humans, told me, “so they aren’t measuring the same thing [in animals] we are measuring in humans.”
Humans, in other words, are complicated: We eat odd foods, drink, take drugs, and stress out. All of these things can affect our response to toxic chemicals, yet none are included even in today’s animal testing. So it is not surprising that past chemical evaluations may have missed some critical toxic responses.
Chlorpyrifos is one of those chemicals for which traditional studies now appear to be insufficient. The insecticide kills the bugs it’s intended to deter by interfering with nerve signaling. Nerve cells constantly chatter with one another, sending chemical messages from nerve to nerve, or nerve to muscle. One such messenger is acetylcholine. Once a muscle cell, for example, is activated by acetylcholine it begins contracting. To stop the activity, the acetylcholine message must then be deactivated, much like the ringer on your phone turning offonce you answer so you can hear to talk. In this case, the enzyme acetylcholinesterase normally deactivates the messenger acetylcholine. But chlorpyrifos inhibits the enzyme, causing unabated signaling and potentially deadly overstimulation. (This is also how the lethal chemical warfare poison Novichok works.)
Toxicologists and regulators can measure the chemical’s effect on signaling to evaluate the impact of exposure to it. The EPA has used these results, along with other information, like when and how a chemical is used, to set food tolerances. Concentrations that don’t cause signaling inhibition, have historically been considered safe.
But part of the problem with setting these kinds of tolerances is that not everyone is affected equally. Studies over the last several decades suggest that infants and children — who have developing brains, maturing metabolic systems, and tend to eat a higher proportion of fruits and vegetables — may be more sensitive to some chemicals than adults. The Food Quality Protection Act of 1996 addressed some of these concerns directing regulators to set standards for children’s health, and to consider the effects of cumulative chemical exposures. These include the additive effects of exposure to chlorpyrifos or similar pesticides, for example, by eating produce like peaches, snap peas, and bell peppers — crops that may be treated with the pesticide.
As the ability to study more subtle impacts of chemicals, particularly in the very young has improved, scientists began questioning if tolerances for chlorpyrifos should be reduced or eliminated because of its impacts on developing brains. “Growing cells are more vulnerable to toxins, and the brain forms over a longer period than do other organs,” wrote Bruce Lanphear, an epidemiologist at Simon Fraser University, in a 2015 paper published in the Annual Review of Public Health. Lanphear argued that to continue to regulate all chemicals as if they have a safe level no longer makes sense, particularly when it comes to protecting children from chemicals that impact brain development. We were taught that “low levels are of no consequence,” says Lanphear, “and we now know that’s not true.”
Chemicals that affect children’s brains, unlike those that injure organs like livers or hearts, affect not just our bodies but who we are, and early deficits are difficult to regain, unless there are serious interventions, says Cory-Slechta. Studies both in the laboratory and in children exposed in utero, suggested that the most recent restrictions on chlorpyrifos were not strict enough. One series of studies by Columbia University perinatal epidemiologist Virginia Rauh and her colleagues revealed an association between chlorpyrifos and intelligence and memorydeficits. The researchers used MRI imaging to find structural changes linked to exposure in the children’s brains. Some of those effects were found at concentrations below those causing acetylcholinesterase inhibition. These studies were just a few of those that convinced EPA regulators in 2015 that chlorpyrifos residues on fruits and vegetables could no longer be considered acceptable.
And then there was a national presidential election, and a new head administrator, Scott Pruitt, was appointed to the EPA. Bedeviled by scandal, Pruitt was forced to resign this summer, but not before postponing a decision on chlorpyrifos regulations until 2022, citing a return to “sound science,” emphasizing uncertainty and trading on doubt. Prior to the agency’s turn-around, former administrator Pruitt met with the CEO of Dow Chemical, which produces chlorpyrifos. Earlier in the year the company had donated $1 million dollars to President Trump’s inaugural activities and spent a total $13.6 million lobbying in 2016.
In May of this year, the state of Hawaii decided not to wait for the EPA, and banned chlorpyrifos. California also began taking another look. Then, this month in a case brought by the original petitioners along with a number of labor groups, the U.S. Court of Appeals for the 9th Circuit, citing the EPA’s failure to determine that exposures through food were safe and the agency’s “patent evasion” of it duties, ordered the agency to do its job and ban the pesticide.
Chemical regulation combines toxicology and other sciences with economics and politics. This is always a tricky process. But our health, and the health of the next generation, require an agency that is receptive to the best available science incorporating new insights into toxic chemicals and values health over profit. Toxicology has certainly advanced, but the federal body responsible for its application, the EPA, currently appears to be in retreat. Pruitt’s departure provides a modicum of hope, as do the federal courts — for now. But, should the agency continue to turn its back on its citizens — and its raison d’être — it will fall to the states, the courts and us, as concerned citizens, to step up.
This post was originally published at UnDark
